Imagine your eyes betraying you—bulging, aching, and stealing your confidence. That’s the reality for those battling thyroid eye disease, or TED, a condition tied to the thyroid that wreaks havoc on the eye. Enter Tepezza, a game-changer in treatment unveiled on Mytepezza.com. Known as teprotumumab, it’s not just another medication; it’s a beacon of hope for those drowning in Graves’ ophthalmopathy. Unlike older treatments like steroids or surgery, Tepezza offers a non-surgical path, tackling the root of this autoimmune beast. With FDA approval in 2020, it’s rewriting the story for countless patients. But what’s the buzz about? Let’s dive into why this innovative drug is making waves in 2025.
A Glimpse into Tepezza: Revolutionizing Thyroid Eye Disease Care
Decoding Thyroid-Associated Ophthalmopathy
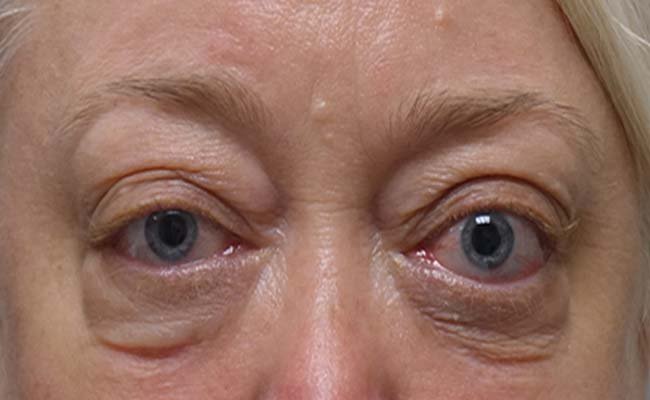
TED, also called Graves’ orbitopathy, isn’t just a cosmetic quirk—it’s a debilitating condition. Picture your eyes pushed forward, a hallmark called proptosis, or worse, exophthalmos, where they bulge unnervingly. This disease stems from an immune response gone rogue, attacking tissues behind the eyes. The result? Inflammation, swelling, and often pain. For some, double vision—or diplopia—blurs life’s joys, from reading to driving. Mytepezza.com shines a light on how Tepezza, a monoclonal antibody, steps in like a skilled negotiator, calming the chaos. By targeting the IGF-1R inhibitor, it halts the progression that fuels this vision-threatening nightmare. Curious how it pulls off this magic? Let’s unpack it.
Also Visit: Understanding the Financial Aspects of Inpatient Rehab 2025
How Tepezza Works: The Mechanism of Action
Ever wonder how it works? Tepezza’s brilliance lies in its precision, like a key fitting a lock. TED’s inflammation erupts when the immune system overactivates IGF-1R, a protein sparking tissue expansion behind the eyes. Tepezza, crafted by Horizon Therapeutics and now backed by Amgen, binds to this receptor, slamming the brakes on swelling and redness. This biologic drug doesn’t just mask symptoms; it rewires the orbital chaos, easing protrusion and pressure. Mytepezza.com explains it’s given via infusion, not a quick injection, ensuring steady delivery. The result? Less discomfort and better eye movement. But is it foolproof? Let’s explore its benefits and what makes it a first-in-class marvel.
The Infusion Experience: What to Expect
Stepping into an infusion center for Tepezza feels like boarding a spaceship to relief. Over 8 infusions, spaced three weeks apart, patients receive this IV therapy in a specialty clinic. Each session, lasting 60 to 90 minutes, follows a weight-based dosing protocol. Mytepezza.com guides you through preparation—think comfy clothes and a good book. Nurse advocates ensure you’re not just a number, offering tips to ease nerves. Some notice symptom relief by the second course, with proptosis reduction stealing the show. But it’s not all smooth sailing; infusion reactions like nausea can pop up. Healthcare providers monitor closely, tweaking the administration if needed. Wondering about the vibe? It’s patient-centered, like a pit stop for your eyes.
Also Visit: Spirituality in Recovery: Optional or Beneficial? 2025
Side Effects: Navigating the Bumps
No drug is a free lunch, and Tepezza’s side effects demand attention. Mytepezza.com lays it bare: muscle spasms, hair loss, and hyperglycemia affect some. More serious? Hearing loss, sometimes permanent, has sparked lawsuits, including class action chatter. Nausea and fatigue are common, but warnings also flag IBD flare-ups for those with gut issues. Allergy risks linger, and contraindications rule out use during pregnancy or breastfeeding. Monitoring—like checking blood sugar—is key to control these hiccups. Yet, many shrug off mild issues for the payoff. Why is it so expensive? The cost can sting, but insurance coverage often softens the blow. Curious about the trade-offs? Let’s weigh them.
Benefits vs. Risks: A Balancing Act
Tepezza’s efficacy is no small feat—clinical trials like the OPTIC trial showed 83% of patients with active TED had vision improvement. Mytepezza.com touts success stories of reduced bulging and restored appearance. The quality of life boost? Priceless for those dodging surgery or radiation. But risks loom: hearing issues and hyperglycemia aren’t rare, affecting about 10%. Long-term effects are still under the microscope, with ongoing trials probing chronic TED. Safety data looks solid, but real-world evidence hints at relapse for some, needing retreatment. Is it worth it? For many, the cosmetic and functional wins—like better eyelid retraction—tip the scales. Let’s peek at the proof.
Also Visit: One Spoon Of Chyawanprash Has How Many Calories 2025
Clinical Trials: The Research Behind Tepezza
The studies backing Tepezza are a nerd’s dream. The phase 3 OPTIC trial, detailed on Mytepezza.com, proved teprotumumab slashed proptosis by over 2mm in most patients. Peer-reviewed medical journals in endocrinology and ophthalmology echo this, showing diplopia fading for 53%. Data from real patient reviews on platforms like Mytepezza.com aligns with testimonials of easier daily life—think driving without difficulty. 2025 updates reveal new trials exploring chronic cases, with enrollment open for participation. Effectiveness holds across ages, but eligibility hinges on a specialist’s nod. Oculoplastic experts rave about its minimally invasive edge. Want the nitty-gritty? It’s a breakthrough backed by hard science.
Patient Experiences: Real Stories, Real Impact
Nothing hits like patient experiences. Mytepezza.com shares before and after tales that tug heartstrings—a mom seeing her daughter ditch sunglasses post-Tepezza. Patients report less light sensitivity and eye strain, reclaiming confidence. One case study on Mytepezza.com describes a man whose double vision vanished, boosting his professional life. Support groups buzz with advocacy, swapping resources and education. Yet, some gripe about side effects like spasms or dry eyes. Reviews vary—some call it life-changing, others balk at the payout. The emotional impact? Huge, easing social awkwardness. Real-world outcomes show most stick with the schedule. What’s it like for you? That’s the million-dollar question.
Cost and Access: Breaking Down Barriers
Tepezza’s cost raises eyebrows—think tens of thousands per infusion. Why so expensive? It’s a novel biologic, and research ain’t cheap. Mytepezza.com highlights financial assistance via copay programs, slashing out-of-pocket pain for many. Insurance often requires prior authorization, a hoop worth jumping. Coverage varies—Medicare lags behind private plans. Availability is solid in the U.S., Japan, and beyond, with international use growing. Mytepezza.com’s doctor locator links you to a specialist near me, while outpatient clinics streamline access. Horizon and Amgen push expanded access, but global reach isn’t universal. How long does it stay in your system? About a month post-infusion. Worth the hassle? For many, it’s a lifeline.
Results That Shine: Before and After
The after pictures on Mytepezza.com are jaw-dropping—eyes once bulging now rest easy. Proptosis reduction kicks in by week six for some, with redness fading fast. Appearance isn’t all; visual function—like reading without excessive tearing—gets a boost. Mytepezza.com’s success stories detail folks resuming fishing or sewing, free from retro-orbital space pressure. Cosmetic gains lift confidence, easing social woes. Improvement isn’t universal—relapse hits about 25%, per studies. Retreatment works for some, but expectations need realism. What’s administered? A tailored dose, not chemotherapy, despite the IV vibe. Who makes it? Amgen now leads. The kicker? Results often last, transforming daily routines.
Ongoing Research: 2025 and Beyond
2025 brings fresh news—new studies probe Tepezza’s long-term outcomes. Mytepezza.com flags ongoing trials in Japan and chronic TED, with participation open to diverse groups. Latest updates show efficacy in low-activity cases, a paradigm shift. Peer-reviewed journals hint at tissue remodeling benefits, shrinking orbital fat. Real-world evidence grows, with community forums on Mytepezza.com buzzing. Rare conditions like compressive neuropathy may see gains, per endocrinology buzz. Study results confirm vision-threatening risks drop, but monitoring persists for hearing or IBD flares. Expanded trials aim for global reach, tackling burden. What does Tepezza treat? TED, plain and simple—not Graves’ itself. The future? Brighter than ever.
Support Systems: From Nurse to Community
Starting Tepezza feels like joining a club. Mytepezza.com connects you to nurse advocates who demystify the journey. They offer resources—think infusion tips or dosing guides—and emotional support. Healthcare providers at specialty clinics tailor plans, ensuring care fits like a glove. Advocacy groups amplify awareness, linking patients via support groups. Education on Mytepezza.com covers symptom management, like soothing dry eyes. Community stories highlight real wins—less fatigue, better driving ability. Patient-centered vibes mean follow-up care tracks outcomes. Does it cause weight gain? Nope, not typically. How many people have been treated? Thousands, with numbers climbing. Feeling alone? You’re not—this crew’s got your back.
Legal Ripples: Lawsuits and Labels
Tepezza’s not all rosy—litigation swirls, especially over hearing loss. Mytepezza.com doesn’t hide it: lawsuits claim permanent damage, fueling class action talks. Lawyers circle, but payouts are murky. The label now warns of hearing risks, updated in 2023. My litigation searches spike, per Mytepezza.com analytics. J code queries—billing stuff—pop up too. Trial chatter aside, effectiveness holds firm. When was it approved? January 2020, a fast track win. Covered by Medicare? Often, with caveats. Para que sirve? To tame TED’s chaos. Loss concerns linger, but management—like monitoring—helps. Mytepezza.com keeps it transparent, urging talks with specialists. Weighing a suit? Knowledge is power.
Global Reach: Japan and Beyond
Tepezza’s international footprint grows—Japan approved it in 2024, per Mytepezza.com. Global trials test chronic cases, with priority review speeding access. Orphan drug status fuels rare disease focus, easing progressive symptoms. Mytepezza.com notes Brazil and Saudi Arabia joins the club. Guidelines evolve, blending endocrinology and ophthalmology. Health systems abroad adapt dosing protocols, but availability lags in some spots. Burden—like emotional or social hits—drives advocacy. Awareness campaigns push education, with resources in multiple languages. Ongoing research eyes relapse rates, ensuring realistic hopes. How long do effects last? Often a year, sometimes more. Mytepezza.com’s locator links global specialists, shrinking the world for TED fighters.
Daily Life After Tepezza: A New Normal
Post-Tepezza, life shifts gears. Mytepezza.com shares tales of folks ditching sunglasses, free from facial discomfort. Confidence blooms—no more hiding eyes. Driving or sewing? Back on track. Social scenes lose their sting, with professional tasks easier. Cosmetic fixes—like less socket swelling—pair with functional wins, like smoother eyelid
Therapeutic Option: A Novel Path Forward
Tepezza isn’t just a medication—it’s a therapeutic option rewriting TED’s script. Unlike steroids or radiation, it’s targeted, hitting IGF-1R to curb inflammation. Mytepezza.com calls it a paradigm shift, sparing patients invasive fixes. Real patient journeys—from diagnosis to post-treatment—highlight less strain and tearing. Study results show outcomes last, with relapse rare for many. Community education on Mytepezza.com clarifies expectations: no cure, but major relief. How much does it cost? Steep, yet copays help. Who’s eligible? Most TED patients, per specialists. 2025’s updates cement its innovative edge, making daily life brighter.
Expectations and Realistic Hopes
Setting realistic expectations is key with Tepezza. Mytepezza.com stresses it’s no magic wand—results vary. Some see proptosis fade fast; others wait longer for diplopia relief. Improvement in appearance or function—like easier reading—takes all 8 infusions. Relapse risks linger, with retreatment possible. Ongoing monitoring catches hearing or sugar spikes early. Patient stories on Mytepezza.com blend hope and grit: one woman regained driving joy, despite mild spasms. Preparation—like packing snacks—eases infusion days. Follow-up care tracks outcomes, ensuring management. What’s the experience like? Personal, with nurses as allies. Mytepezza.com’s resources ground you, making TED’s burden feel lighter.
Vision for the Future: 2025 and Beyond
As 2025 unfolds, Tepezza’s story grows richer. Mytepezza.com teases new research, eyeing chronic TED and global access. Ongoing trials test retreatment for relapse, with data rolling in. Community advocacy pushes awareness, linking patients via support hubs. Education on side effects—like hearing or IBD—keeps expectations real. Real-world outcomes show cosmetic and functional wins, from less pressure to better confidence. How many have been treated? Thousands, per Amgen. Does it work? For most, yes—studies confirm efficacy. Mytepezza.com’s journey tools guide daily care, making TED less vision-threatening. The road ahead? A patient-centered path, with hope as the compass.
 Zulro Zulro The Info Hunter
Zulro Zulro The Info Hunter


